Which of the Following Indicates an Endothermic Reaction
Converting frost to water vapor melting boiling and evaporation in general are endothermic processes. To determine whether reaction is extra chronic or undergone IQ.

The Picture Above Is Showing An Example Of Endothermic Because It S Taking Energy From The Things Around It W Chemistry Physical Chemistry Exothermic Reaction
This also means that a decrease in temperature occurs with absorption of heat.
. AB C D heat C. Therefore temperature of the system decreases. 13 Specific heat capacity.
Suppose we place two moles each of S O 2 and O 2 in the reaction vessel at 2 5 C and adjust the volume to give a total pressure of 1. Using standard reduction potentials predict whether the following metals can be reduced to the metallic state by hydrazine under standard conditions in. 3 Get Other questions on the subject.
7 Liquid Z C 353 Jg-C. A reaction where heat energy is absorbed by the molecules of a substance is known as an endothermic reaction. In an endothermic reaction heat being absorbed is utilized to break the bonds.
If a solution had a ph of 7 what is the solution. Indicate whether each of the following reactions is endothermic or exothermic. Correct answer - Question 7 of 10 Which of the following indicates an endothermic reaction.
A5000 g sample of niso4 h2o decomposed to give 2755 g of anhydrous niso4. KJmol Toitwool adolorib919 reaction coordinate reaction coordinate reaction coordinate reaction coordinate yam 2692 iw noite91 i r tobas 16101 DA to ngiz adib919. ΔG will decrease with raising the temperature.
𝐻 1435 kJ 1254 kJ 181 kJ N 2 g O 2 g 2 NOg 𝐻 181 kJ This method would be extremely limited if we were restricted to the use of bond dissociation energies for diatomic molecules. Since more energy is required to break bonds than is released by bond formulation the reaction is endothermic. If there is a drop in temperature ofsolution ie.
Another question on Chemistry. For example The value of ve for an endothermic reaction. Question 7 of 10 Which of the following indicates an endothermic reaction.
0 a t m. Which of the following indicates an endothermic reaction. A B heat C.
Which of the following energy diagrams shows a concerted endothermic reaction. The value of is positive for an endothermic process. Less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst.
ΔG will be positive. V A reaction that is spontaneous in the forward direction is non spontaneous in the reverse direction. In endothermic reactions the products have more energy than the reagents this means that they do not occur exponentially.
A B heat C D heat. The following is a representation of the chemical equation. False v An endothermic reaction with positive entropy change can be spontaneous only at low temperatures v A spontaneous reaction may be either endothermic or exothermic.
AB C D heat C. 4 NH3 g 5 02 g 4NO2 g 6H2O I DHrxn -11692 kJ 3D C. - The direction of the arrow in an enthalpy diagram indicates whether a reaction is exothermic or endothermic - In an endothermic reaction the reactants will be at the bottom of the enthalpy diagram Match each type of calorimeter with the thermochemical property that it measures.
A key indication of an endothermic reaction is if the reactionvessel is itself cold ie. Example of a temperature change that might occur in an endothermic reaction. Whereas when energy is released by the molecules of a substance in a reaction then it is known as an.
ΔG will increase with raising the temperature. The reaction is ignited by a spark and goes to completion. An endothermic reaction for which the system exhibits an increase in entropy.
Which describes an endothermic reaction. 15 Energy stored in the bonds of a substance C H bonds contain a. Consider the following reaction taking place in a container fitted with a movable piston.
An endothermic reaction occurs when ammonium chloride NH 4 Cl is dissolved in water. NH 4 Cl s H 2 O l NH 4 Cl aq Heat. C s 02 g Co2 g DHrxn 9405 kcal b.
Which of the following indicates that an endothermic reaction has occurred. The ammonium NH 4 and chloride Cl ions separate from the salt. This means that energy is required to be inputted in order to achieve this.
We have to see if the Gibbs Free Energy also known as Delta G value for that reaction is positive or negative when Delta G is positive or its above zero. 2 PCI3 1 O2 g OPCI3 1 DHrxn -3251 kJ. Endothermic reactions are those that absorb energy in the form of heat.
Why is heat generated when methanol dissolves in water. ΔG will be negative. A chemical reaction where heat energy is absorbed by the reactant molecules is known as an endothermic reaction.
Instead the system is extended by deriving approximate bond. If an exothermic reaction is reversed it becomes an endothermic reaction. 12q m C ΔT.
10Energy will flow from substance A to substance B. In an exothermic reaction the bonding energy of the product is. Indicate whether the following statements are true or false.
What is the formula of the hydrate. The end result our products with Mawr Energy than the starting reactant.
In An Endothermic Reaction The Energy Of Products Is More Than The Reactants Means They Have More Heat I Have Assumed More Energy To Be More Heat Energy So Why Does The

Heat Of Reaction Reflects The Difference In Enthalpy Between The Products And The Reactants Teaching Chemistry Chemistry Education Teaching Science

Endothermic Lettering System Energy
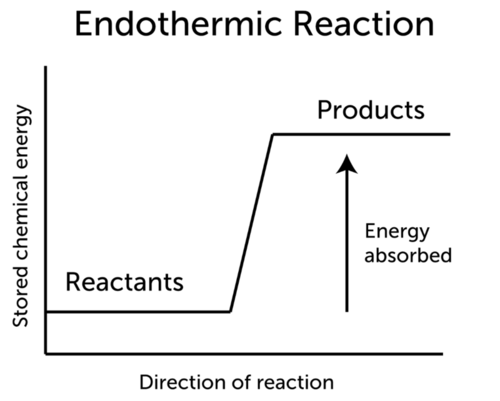
Endothermic Reaction Ck 12 Foundation

Endothermic Vs Exothermic Reactions Chemtalk

Schematic Representation Of The Energy Level Diagram Of An Exothermic Download Scientific Diagram

H Is A State Function Because E P V Are State Functions So It Depends Only On The Difference Betwee Chemistry Education Teaching Chemistry Science Chemistry

Endothermic Exothermic Reactions Energy Changes In Chemical Reactions Mcat Content
In An Endothermic Reaction Is All Of The Activation Energy Absorbed If Not Can An Endothermic Reaction Release Any Heat If So Why Isn T It Exothermic Quora
What Is The Sign Convention For Enthalpy For Exothermic And Endothermic Reaction Quora

Kinetic And Potential Energy Worksheet Key Kinetic And Potential Energy Worksheet Key In An Understanding Medium Can Be Used To Try Students Talents And Under
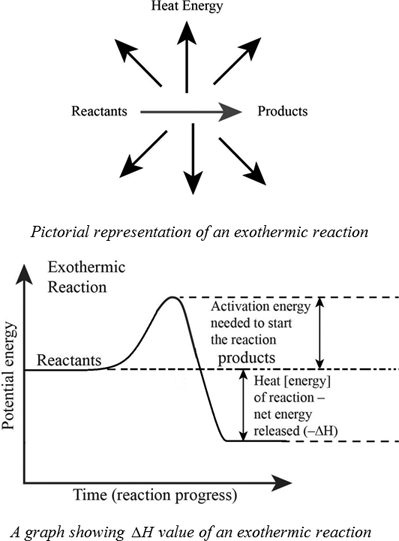
Definition Of Exothermic And Endothermic Reactions Chegg Com

Energy Diagrams For The Transfer Of Internal Energy Between A System And Its Surroundings The Change I Chemistry Education Chemistry Classroom Internal Energy

Endothermic Vs Exothermic Reactions Chemtalk

Endothermic Or Exothermic Reaction Of The Vo 2 Phase Caused By Phase Download Scientific Diagram
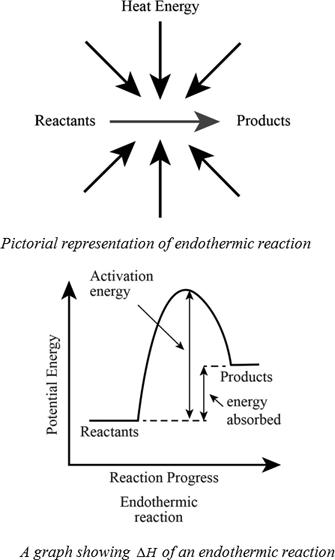
Definition Of Exothermic And Endothermic Reactions Chegg Com



Comments
Post a Comment